
The Essential Role of Metals in Modern Technology and Sustainability
Understanding the Pivotal Role of Metals in Today's World
Customised advise from one of our specialists.
Fill in the form below and we will contact you as soon as possible.

From Everyday Use to High-Tech Applications
The Ubiquity of Metals

Metals have become indispensable in our current world. From building and construction materials, various means of transport to electronics and household items such as cooking utensils. We unconsciously use metal objects every day without realizing what it takes before the metal is processed into a utensil.
The production of solar panels, electric cars, and wind turbines in particular requires enormous quantities of various metals, often much more than the use of fossil fuels. This includes rare metals such as cobalt, lithium, and neodymium, but the need for aluminum, cadmium, copper, gallium, indium, graphite, iron, lead, nickel, silica, tin, and zinc is also increasing sharply. Due to the enormous scale of solar and wind farms and electric cars, the need for these types of metals has also increased explosively.

Exploring the Different Types of Metals
Ferrous, Non-Ferrous, and Heavy Metals

A distinction is made between ferrous metals and non-ferrous metals. Iron and all iron-based alloys, of which steel is the best known, are called ferrous metals, the other metals, such as aluminum, gold, and copper, are non-ferrous metals.
In addition, the term heavy metals is also regularly used. These include lead, mercury, copper, chromium, nickel, and cadmium. Metal compounds include cadmium chloride, mercury dichloride and lead chromate. Heavy metals have many applications but are mainly used to refine metals, where the treated materials acquire certain desired properties. Heavy metals are, for instance, also used in batteries.
Get in touch with us

The Journey from Ore to Metal
Extraction and Refining Processes

Metals are rarely found in pure form in nature. Metals are usually mined in the form of ores, which are converted into the pure metal form in metallurgy. Examples of this are the extraction of cast iron from iron ore using blast furnaces or the electrochemical conversion of bauxite into aluminum.
Manufacturing pure metals from their ore is, in many cases, a cost- and energy-intensive process. Each metal has its own kind of process. As is known, iron is by far the most common metal and needs heavy industry to convert it into steel. Different processes, from the blast furnace, oxygen furnace converter, hot and cold rolling mills, up to the hot dip galvanizing line, are necessary for a usable end product.
Other metals like aluminum are extracted from alumina by electrolysis. Alumina is purified bauxite, which is basically aluminum ore. The process of electrolysis involves using an awful lot of electricity to break down electrolytes to form base elements. To make this process possible the alumina must be liquified before the electrolysis to let the electricity pass. Aluminum is one of the metals which is extracted from its ore by this method.
Zinc is made by using a combination of a smelting process and an electrolytic process. A mixture of zinc-containing concentrates or secondary zinc material, such as zinc oxides, is used as raw material for the roasting plant. The concentrate is burned, releases impure zinc oxide, sulfur dioxide and heat. The impure zinc oxide is dissolved in sulfuric acid to form a zinc sulfate solution. The contaminants, like other metals, are removed from the solution as much as possible. In the final phase, zinc metal is extracted from the purified zinc sulphate solution by means of electrolysis.
Copper ore first undergoes a flotation process to purify the ore, this is necessary because copper ore normally contains less than 1% copper. This process releases various types of other metals, ranging from nickel and cobalt to various precious metals. The ore is then further purified using a roasting process, and copper concentrate is converted and concentrated to a higher purity via a converter, which is basically a chemical process. A final step towards pure copper is achieved by electrolytic refining.
Mining and Metallurgy
Assessing the Environmental Footprint

Mining results in large quantities of ores being excavated and then transported to industrial processing plants, where the metal is extracted from the ore. Ultimately, unusable ore residues remain and much rinse water is polluted. There is considerable clearing of areas where ores are mined to exploit the ore-rich soil. Infrastructure must also be constructed to transport the ores. This consumes a lot of energy, consumes a lot of water, creates air pollution, and releases toxic waste products.
These are some examples of how various ores are ultimately converted into a usable semifinished product via thermal, electrolytic, and chemical means. These processes require an enormous amount of energy and, in the past, had a major impact on the immediate environment in the form of the precipitation of particulate matter. They also emit large amounts of SOx, NOx, CO2 and, in some cases, heavy metals and various chemical components.
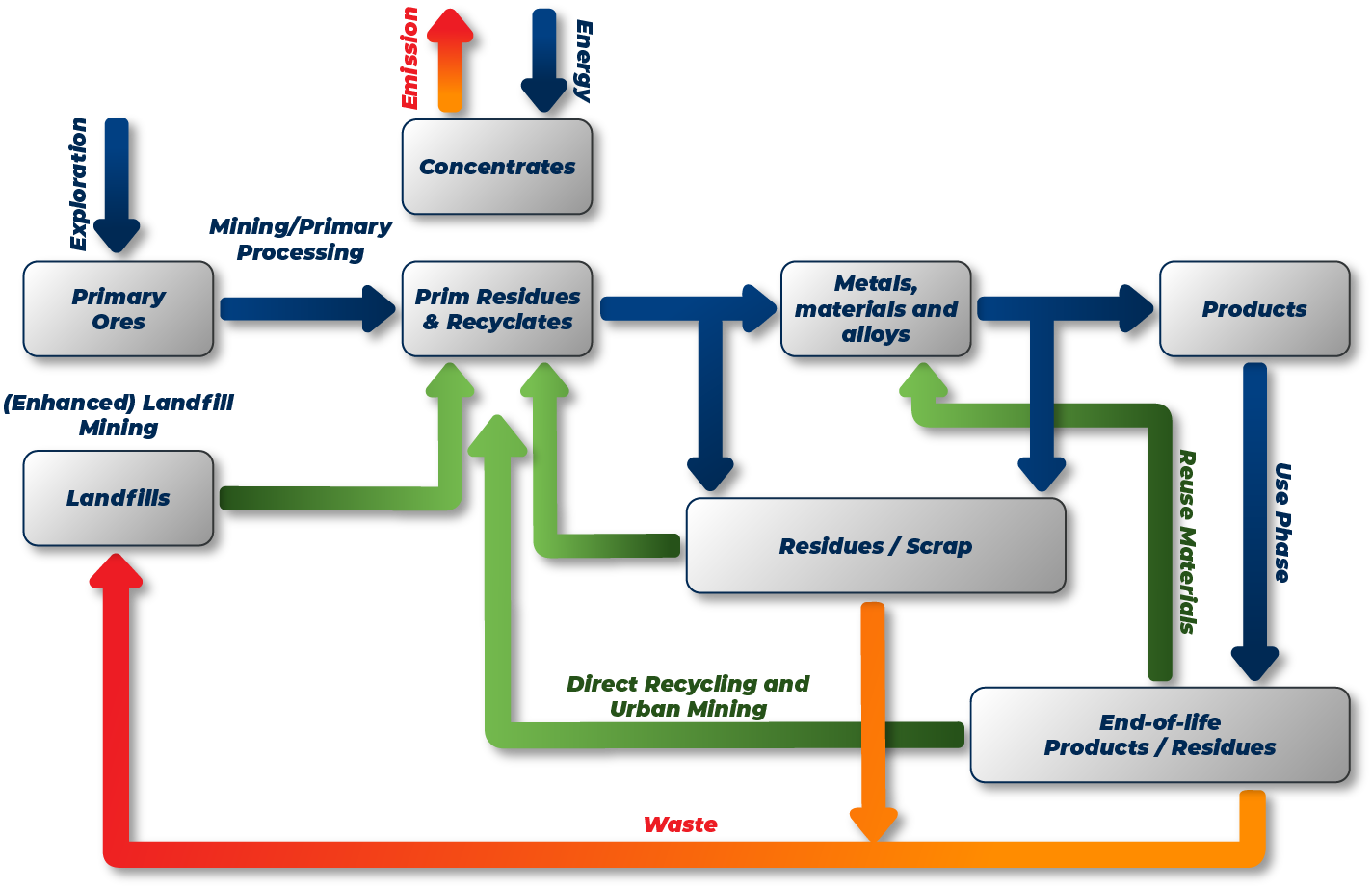

Metal Recycling
The Importance and Benefits of Metal Recycling for the Environment

The benefits of metal recycling are enormous; reuse of these types of materials is crucial to prevent soil depletion. Global supplies of metal ore are also limited and finite. By reusing metals, no ore needs to be extracted. This is, of course, beneficial for the living environment and nature. Processing extracted ore into reusable metal saves a huge amount of energy: for ferrous metals, this can be up to 75%; for aluminum, even 95%; for zinc, this is still 60%; and for copper, approximately 85%.
In a world where climate change and carbon dioxide emissions are central every day, energy savings are, of course, the most important reason for recycling metals. By reusing and recycling metal and iron materials, gains are also made in this area, and the burden on the environment and air quality is reduced. Consider the following average savings: up to 86% less air pollution, 40% reduction in water use, and therefore more or less 76% less water pollution. Finally, the major advantage of metal recycling is that this material can be recycled virtually 100%. So little to no material is lost during the recycling process.
Game-changing benefits
Discover the game-changing benefits of scrubbers and propel your business forward with our free whitepaper download.

Advanced Wet Gas Scrubbers for Emission Reduction in Metal Industry
Particulate and Chemical Emission Control

According to the Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control) for ferrous and non-ferrous metal production and processing, scrubbers are among the best available techniques to reduce emissions. Ravebo has designed wet gas scrubbers to absorb and reduce virtually all common emissions in the metal industry. The scrubbers are designed according to a so-called open spray system, in which the absorption takes place in the very fine droplets generated by special nozzles. The big advantage, aside from high efficiency, of this type of scrubber is a very low pressure drop, which means that energy consumption is low.
Another strong point of this type of scrubber is its ability to decimate both particulate matter and chemical components simultaneously to acceptable emission limits. The scrubbers are so versatile that the installation can be constructed from several sections in series so that the entire spectrum of contaminants can be removed and the temperature of the waste gas can be drastically reduced through adiabatic cooling. The construction and materials used in the scrubber have been developed in such a way that incoming gas temperatures of up to 900 °C can be used.
The scrubber actually converts a gas-side problem into a liquid-side problem. The contaminants carried by the off gas are absorbed by the washing liquid, causing it to become contaminated. To avoid draining a lot of water to stay within the emission limit values on the gas side, facilities have been installed that allow the water to be recycled.

Ravebo's Gas Analyzers
Advanced Gas and Dust Analyzers for Emission Monitoring in Industry

According to the Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control) for ferrous and non-ferrous metal production and processing, scrubbers are among the best available techniques to reduce emissions. Ravebo has designed wet gas scrubbers to absorb and reduce virtually all common emissions in the metal industry. The scrubbers are designed according to a so-called open spray system, in which the absorption takes place in the very fine droplets generated by special nozzles. The big advantage, aside from high efficiency, of this type of scrubber is a very low pressure drop, which means that energy consumption is low.
Another strong point of this type of scrubber is its ability to decimate both particulate matter and chemical components simultaneously to acceptable emission limits. The scrubbers are so versatile that the installation can be constructed from several sections in series so that the entire spectrum of contaminants can be removed and the temperature of the waste gas can be drastically reduced through adiabatic cooling. The construction and materials used in the scrubber have been developed in such a way that incoming gas temperatures of up to 900 °C can be used.
The scrubber actually converts a gas-side problem into a liquid-side problem. The contaminants carried by the off gas are absorbed by the washing liquid, causing it to become contaminated. To avoid draining a lot of water to stay within the emission limit values on the gas side, facilities have been installed that allow the water to be recycled.
Get in touch with our scrubber specialist
Our gas scrubbers with analyzing systems make it possible to clean various gas flows. This leads to a cleaner living environment and more sustainable production processes. Curious about the possibilities? Our specialists are happy to provide you with appropriate advice.
